
Researchers unravel how the immune system responds to diffuse cutaneous leishmanisis
In diffuse cutaneous leishmanisis (DCL), a rare form of leishmaniasis, parasites grow uncontrolled in skin lesions across the body. For the first time, researchers reporting in PLOS Neglected Tropical Diseases have now profiled how the human immune system responds to a DCL infection and, in turn, how Leishmania amazonensis adapts to the human host.
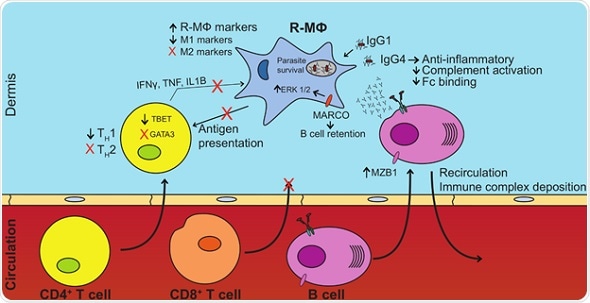
Aspects of B cell, T cell, and macrophage responses contribute to parasite survival in DCL patients. Increased B cell presence and altered antibody repertoires promote anti-inflammatory phenotypes in the lesion microenvironment. This nullifies parasite killing, and possibly promotes the diffuse nature of the disease. Increased anti-inflammatory factors also negate TH1 cell activation, cytotoxic T cell infiltration, activation, and T cell effector functions necessary for clearance of intracellular pathogens. Credit: Christensen, et al. (CC BY 4.0, 2019)
American tegumentary leishmaniasis— the broad name for parasitic infections with Leishmania pathogens in the Americas—affects between 0.7 and 1.2 million people a year. Those infections present in one of four categories, depending on their severity and localization; DCL is one of those categories. L. amazonensis, one of two major Leishmaniaparasites in Brazil, can in rare cases manifest as DCL, with lesions across the skin.
To better understand how this rare form of the infection occurs, David Mosser, of the University of Maryland, College Park, and colleagues analyzed material collected from biopsies of 6 DCL patients in Brazil. Samples included both healthy tissue and L. amazonensis-infected lesions. In each sample, the researchers sequenced genetic transcripts—reflecting the expression patterns of genes—from both human cells and parasites.
The team found a heightened presence of B cell transcripts—rather than T cells— in the host response to L. amazonensis. The observations also pointed to patterns of human macrophage gene activation in DCL lesions similar to regulatory macrophages, with higher levels of 90 regulatory genes and lower levels of 146 inflammatory genes compared to macrophages from localized leishmaniasis.
On the parasite side, they observed that patterns of gene expression more closely resembled those seen in parasites growing in quiescent macrophages, in vitro, than those in localized lesions under T cell pressure. 280 parasite genes in DCL and 336 parasite genes in LCL exhibited patterns unique to their disease manifestation. Lastly 262 parasite genes were commonly highly expressed regardless of disease and condition.
“This work presents an in-depth assessment of the host and parasite transcriptomes in the rare disease diffuse cutaneous leishmaniasis,” the researchers say. “These studies on parasite gene expression may reveal new targets for vaccine development in this neglected tropical disease.”
Source:
No comments:
Post a Comment