A new DRUG TRIALS SNAPSHOT is now available.

AYVAKIT is used to treat adult patients with gastrointestinal stromal tumor (GIST) whose disease:
- is caused by certain abnormal platelet-derived growth factor receptor alpha (PDGFRA) genes and,
- cannot be surgically removed or,
- has spread throughout the body (metastatic GIST).
GIST is type of stomach, bowel, or esophagus tumor.
AYVAKIT is a tablet taken once daily on an empty stomach.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots: UBRELVY
UBRELVY (ubrogepant)
you-brel-vee
Allergan
Approval date: December 23, 2019
you-brel-vee
Allergan
Approval date: December 23, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
UBRELVY is a drug used for the acute treatment of migraine with or without aura in adults.
A migraine is a type of headache that, in addition to pain, can be associated with nausea, vomiting, and sensitivity to light or sound. Some patients may experience an aura, which may consist of temporary visual, or other symptoms, shortly before the onset of the headache.
How is this drug used?
UBRELVY is a tablet taken by mouth as needed. A second tablet may be taken two hours after the first one, if needed.
What are the benefits of this drug?
A higher percentage of patients did not have a headache two hours after taking UBRELVY compared to patients who took a placebo. Also, a higher percentage of patients who took UBRELVY did not have their most bothersome migraine-associated symptom (e.g., light sensitivity, sound sensitivity, or nausea) two hours after taking the drug, in comparison to patients who received placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: UBRELVY worked similarly in men and women.
- Race: UBRELVY worked similarly across racial groups.
- Age: UBRELVY worked similarly among patients younger and older than 40 years of age. The number of patients 65 years and older was limited; therefore, differences in how UBRELVY worked between patients younger and older than 65 years of age could not be determined.
What are the possible side effects?
The most common side effects of UBRELVY are nausea and sleepiness.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar across racial groups.
- Age: The occurrence of side effects was similar among patients younger and older than 40 years of age. The number of patients 65 years and older was limited; therefore, differences in side effects between patients younger and older than 65 years of age could not be determined.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved UBRELVY based on evidence from two clinical trials (Trial 1/NCT02828020 and Trial 2/NCT02867709) of 2423 patients with migraine headaches. Trials were conducted at 188 sites in the United States.
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Demographics by Sex (safety population)
Adapted from FDA Clinical Review
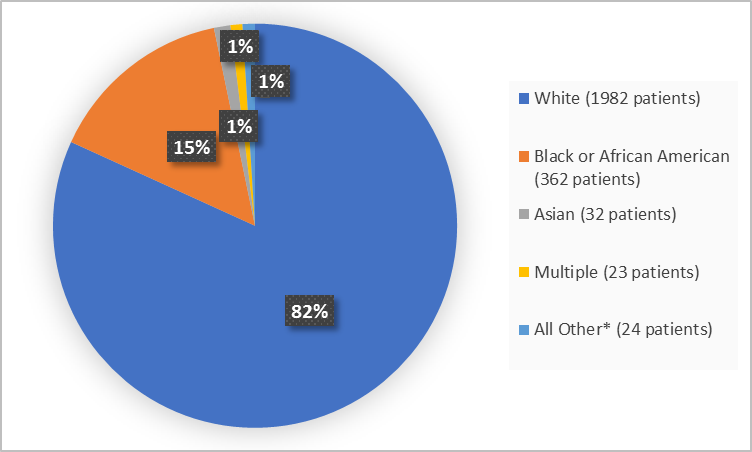
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Demographics by Race (safety population)
* All Other includes American Indian or Native Alaskan, and Native Hawaiian or Other Pacific Islander
Adapted from FDA Clinical Review
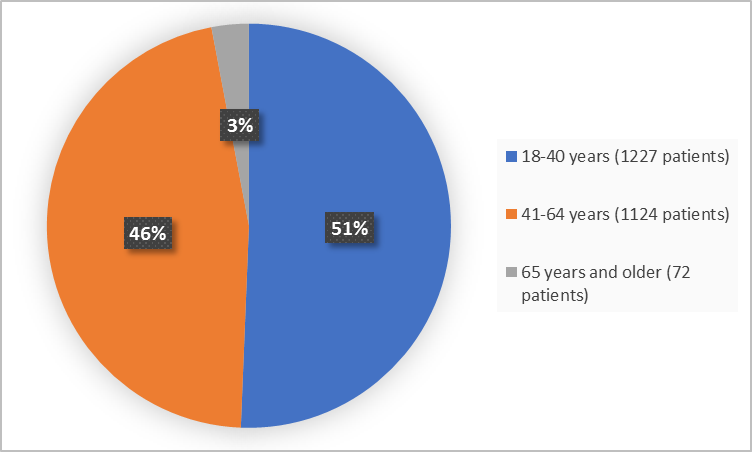
Figure 3 summarizes the percentage of patients by age in the clinical trials
Figure 3. Demographics by Age (safety population)
Adapted from FDA Review
How were the trials designed?
The efficacy and safety of UBRELVY were evaluated in two trials of patients with migraine headache with or without aura.
Patients were instructed to treat moderate to severe migraine headache pain with one dose of UBRELVY or placebo. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed. A second dose of the same medication or the patient’s usual treatment for migraine was allowed between 2 to 48 hours after the initial treatment.
The benefit of UBRELVY was assessed based on the percentage of patients who became pain free within 2 hours and comparing it with placebo treated patients. The assessment also included the percentage of patients who were free of their associated most bothersome migraine symptom within 2 hours of taking the trial drug.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.

No comments:
Post a Comment