
Volume 25, Number 5—May 2019
Dispatch
Neonatal Conjunctivitis Caused by Neisseria meningitidis US Urethritis Clade, New York, USA, August 2017
On This Page
Cecilia B. Kretz1 , Genevieve Bergeron1, Margaret Aldrich, Danielle Bloch, Paula E. Del Rosso, Tanya A. Halse, Belinda Ostrowsky, Qinghuan Liu, Edimarlyn Gonzalez, Enoma Omoregie, Ludwin Chicaiza, Greicy Zayas, Bun Tha, Angela Liang, Jade C. Wang, Michael Levi, Scott Hughes, Kimberlee A. Musser, Don Weiss, and Jennifer L. Rakeman
, Genevieve Bergeron1, Margaret Aldrich, Danielle Bloch, Paula E. Del Rosso, Tanya A. Halse, Belinda Ostrowsky, Qinghuan Liu, Edimarlyn Gonzalez, Enoma Omoregie, Ludwin Chicaiza, Greicy Zayas, Bun Tha, Angela Liang, Jade C. Wang, Michael Levi, Scott Hughes, Kimberlee A. Musser, Don Weiss, and Jennifer L. Rakeman
Abstract
We characterized a case of neonatal conjunctivitis in New York, USA, caused by Neisseria meningitidis by using whole-genome sequencing. The case was a rare occurrence, and the isolate obtained belonged to an emerging clade (N. meningitidis US nongroupable urethritis) associated with an increase in cases of urethritis since 2015.
Neonatal conjunctivitis caused by Neisseria meningitidis is a rare occurrence. Using whole-genome sequencing, we analyzed an isolate of N. meningitidisfrom a 3-day-old infant in New York, USA, who was given a diagnosis of unilateral conjunctivitis.
On August 31, 2017, a 3-day-old infant was given a diagnosis of unilateral conjunctivitis caused by Neisseria meningitidis. The infant was born in a New York City, New York, USA, hospital to a healthy mother by cesarean section performed for prolonged rupture of membranes. A conjunctival swab specimen isolated N. meningitidis, which was identified by real-time PCR as nongroupable N. meningitidis. Nongroupable N. meningitidis lacks capsule production, which is a major virulence factor in causing invasive meningococcal disease. The infant received erythromycin gonococcal ophthalmic prophylaxis according to standard care guidelines and bacitracin/polymyxin ointment for empiric treatment of conjunctivitis and was discharged to home.
When N. meningitidis was identified on day 4 of life, the infant was hospitalized and underwent a sepsis workup that included blood and cerebrospinal fluid cultures (both showed negative results). Upon review of the limited literature available on primary meningococcal conjunctivitis, we determined that, although the conjunctivitis had improved, a 5-day course of therapy with parenteral ceftriaxone was warranted. The infant was discharged on day 8 of life.
N. meningitidis is a rare but known cause of conjunctivitis in newborns. A case of N. meningitidis perinatal transmission linked to genital and nasopharyngeal parental colonization has been described (1). In the case-patient described in our study, early onset of illness suggests that the infant might have acquired the infection intrapartum from maternal N. meningitidis vaginal colonization.
Because of concerns for parental N. meningitidis colonization and possible future transmission to the newborn, we offered meningococcal postexposure chemoprophylaxis to the parents. The mother received 1 dose of ciprofloxacin, which is one of the options for meningococcal postexposure chemoprophylaxis. Maternal nasopharyngeal and vaginal cultures were obtained 3 weeks after the infant’s diagnosis and maternal chemoprophylaxis, at which time the mother was able to be evaluated. Maternal cultures showed negative results. The infant’s father declined to provide screening cultures.
Whole-genome sequencing performed at the New York City Public Health Laboratory showed that the conjunctival isolate had specific molecular characteristics found in the emerging US N. meningitidis nongroupable urethritis clade associated with an increase in cases of urethritis in Ohio, Michigan (2,3), and other locations (4). This novel clade belongs to sequence type 11/clonal complex 11, a hyperinvasive lineage that has caused large meningococcal outbreaks (5,6).
Some specific characteristics found in urethritis-associated N. meningitidis genomes include multigene deletion at the capsule locus, causing its nongroupable phenotype, and complete acquisition of a functional norB–aniA gene cassette, a gonococcal-acquired gene suspected to enable survival in anaerobic environments (7). The aniA gene is commonly found in N. meningitidis. However, many meningococcal strains have frameshift mutations in the operon.
We performed antimicrobial drug susceptibility tests for the isolate by using Etest and obtained results similar to those for other isolates of the N. meningitidis urethritis clade (3). The isolate was sensitive to azithromycin, cefotaxime, ceftriaxone, chloramphenicol, ciprofloxacin, levofloxacin, meropenem, minocycline, rifampin, and trimethoprim/sulfamethoxazole and had intermediate susceptibility to penicillin and ampicillin.
We used whole-genome–based single nucleotide polymorphism (SNP) to compare the isolate from the infant with 9 publicly available N. meningitidis urethritis sequences (PubMLST, https://pubmlst.org) (4,8) and 48 meningococcal sequences from New York City that belonged to sequence type 11. These sequences were from isolates obtained from invasive cases detected by surveillance: nonsterile sources, such as urethral, anal, and oropharyngeal specimens obtained during a men who have sex with men (MSM) carriage study conducted in 2016.
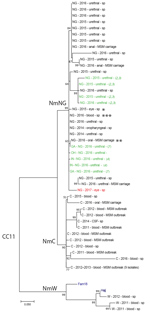
Figure. Molecular phylogenetic analysis of Neisseria meningitidis based on whole-genome sequence data from New York City, New York, USA, and publicly available sequences belonging to the multilocus sequence typing group cc11. Isolates...
Genomic analyses confirmed that the conjunctivitis isolate was phylogenetically part of the N. meningitidis urethritis clade previously described (2,4,7) (Figure) because it clustered with available sequences that had only 20–50 SNP differences. This clade was composed mostly of urethral and anal isolates. However, 1 other conjunctivitis-associated isolate from 2015 that was identified from an immunosuppressed 43-year-old woman in New York City (Figure) and an oropharyngeal isolate collected during the MSM carriage study in 2016 (S. Ngai et al., pers. comm.) (Figure) were also part of the N. meningitidis urethritis clade. One isolate from the N. meningitidis urethritis clade had caused invasive meningococcal disease in a healthy, previously vaccinated, non-MSM man who had a history of childhood meningitis (Figure).
These findings are unusual because only a few invasive isolates have been described as part of the N. meningitidis US nongroupable urethritis clade (8). These observations demonstrate that strains belonging to this clade can cause invasive disease. However, additional information on these cases to characterize risk factors is limited. Although surveillance of N. meningitidis is primarily focused on invasive disease, N. meningitidis has been reported from noninvasive sites, such as urethral and oropharyngeal sites. In addition, few cases of primary meningococcal conjunctivitis are reported in the literature.
Our study showed that an N. meningitidis isolate belonging to the sequence type 11/clonal complex 11 clade with multigene deletion at the capsule locus has likely caused neonatal conjunctivitis in this child. Strains from this clade have the genetic potential to cause invasive meningococcal disease. The organism was not recovered from the infant’s mother. However, she was not tested until 3 weeks after identification of the N. meningitidis isolate and after postexposure prophylaxis. The infant’s infection might have been healthcare associated, but was more likely acquired by maternal genitourinary colonization ascending to the amniotic fluid during the period of prolonged rupture of membranes.
Modes of transmission are not fully understood for the N. meningitidis US nongroupable urethritis clade. Given its potential to cause invasive disease, more studies are needed to increase our understanding and to inform control and prevention measures. A better understanding of N. meningitidisnoninvasive disease, including conjunctivitis in newborns and urethritis, will improve our understanding of the clinical manifestation, transmission modes, and epidemiologic evolution of N. meningitidis strains.
Dr. Kretz is a molecular microbiologist, bioinformatician and laboratory leadership service fellow at the Centers for Disease Control and Prevention who is assigned to the New York City Department of Health and Mental Hygiene, Queens, NY. Her primary research interests are molecular characterization and comparative genomics, using whole-genome sequencing, of respiratory pathogens, such as N. meningitidis and Legionella spp.
Acknowledgments
We thank Justine Edwards and the Bacterial Meningitis Laboratory, Meningitis and Vaccine Preventable Disease Branch, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, and the Antimicrobial Resistance and Characterization Laboratory, Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention for participating in this study.
This study was supported by an Epidemiology and Laboratory Capacity for Infectious Diseases Cooperative Agreement from the Centers for Disease Control and Prevention.
References
- Chacon-Cruz E, Alvelais-Palacios JA, Rodriguez-Valencia JA, Lopatynsky-Reyes EZ, Volker-Soberanes ML, Rivas-Landeros RM. Meningococcal neonatal purulent conjunctivitis/sepsis and asymptomatic carriage of N. meningitidis in mother’s vagina and both parents’ nasopharynx. Case Rep Infect Dis. 2017;2017:6132857.
Figure
Cite This ArticleOriginal Publication Date: 3/26/2019
1These authors contributed equally to this article.
No comments:
Post a Comment