
Volume 25, Number 5—May 2019
Research
Management of Central Nervous System Infections, Vientiane, Laos, 2003–2011
On This Page
Audrey Dubot-Pérès , Mayfong Mayxay, Rattanaphone Phetsouvanh1, Sue J. Lee, Sayaphet Rattanavong, Manivanh Vongsouvath, Viengmon Davong, Vilada Chansamouth, Koukeo Phommasone, Catrin Moore, Sabine Dittrich, Olay Lattana, Joy Sirisouk, Phonelavanh Phoumin, Phonepasith Panyanivong, Amphonesavanh Sengduangphachanh, Bountoy Sibounheuang, Anisone Chanthongthip, Manivone Simmalavong, Davanh Sengdatka, Amphaivanh Seubsanith, Valy Keoluangkot, Prasith Phimmasone, Kongkham Sisout, Khamsai Detleuxay, Khonesavanh Luangxay, Inpanh Phouangsouvanh, Scott B. Craig, Suhella M. Tulsiani, Mary-Anne Burns, David A.B. Dance, Stuart D. Blacksell, Xavier de Lamballerie, and Paul N. Newton
, Mayfong Mayxay, Rattanaphone Phetsouvanh1, Sue J. Lee, Sayaphet Rattanavong, Manivanh Vongsouvath, Viengmon Davong, Vilada Chansamouth, Koukeo Phommasone, Catrin Moore, Sabine Dittrich, Olay Lattana, Joy Sirisouk, Phonelavanh Phoumin, Phonepasith Panyanivong, Amphonesavanh Sengduangphachanh, Bountoy Sibounheuang, Anisone Chanthongthip, Manivone Simmalavong, Davanh Sengdatka, Amphaivanh Seubsanith, Valy Keoluangkot, Prasith Phimmasone, Kongkham Sisout, Khamsai Detleuxay, Khonesavanh Luangxay, Inpanh Phouangsouvanh, Scott B. Craig, Suhella M. Tulsiani, Mary-Anne Burns, David A.B. Dance, Stuart D. Blacksell, Xavier de Lamballerie, and Paul N. Newton
Abstract
During 2003–2011, we recruited 1,065 patients of all ages admitted to Mahosot Hospital (Vientiane, Laos) with suspected central nervous system (CNS) infection. Etiologies were laboratory confirmed for 42.3% of patients, who mostly had infections with emerging pathogens: viruses in 16.2% (mainly Japanese encephalitis virus [8.8%]); bacteria in 16.4% (including Orientia tsutsugamushi [2.9%], Leptospira spp. [2.3%], and Rickettsia spp. [2.3%]); and Cryptococcus spp. fungi in 6.6%. We observed no significant differences in distribution of clinical encephalitis and meningitis by bacterial or viral etiology. However, patients with bacterial CNS infection were more likely to have a history of diabetes than others. Death (26.3%) was associated with low Glasgow Coma Scale score, and the mortality rate was higher for patients with bacterial than viral infections. No clinical or laboratory variables could guide antibiotic selection. We conclude that high-dependency units and first-line treatment with ceftriaxone and doxycycline for suspected CNS infections could improve patient survival in Laos.
Central nervous system (CNS) infections, which can be caused by a number of different viruses, bacteria, fungi, and parasites, cause substantial disease and death in Southeast Asia (1). The etiologies of these infections are usually confirmed in <50% patients globally (2,3). Conventionally, most CNS infections are classified as meningitis or encephalitis by using a diverse set of clinical and laboratory definitions. The main causes of meningitis reported in Asia are Mycobacterium tuberculosis, Streptococcus pneumoniae, Streptococcus suis, Neisseria meningitidis, and Cryptococcus spp. (Appendix Table 1). Physicians rarely consider rickettsial and leptospiral pathogens, but interest in these reemerging treatable etiologies is resurfacing (4). Emerging viruses are important causes of CNS infections in Asia. Japanese encephalitis virus (JEV) causes ≈68,000 cases of encephalitis a year (5), and dengue virus is increasingly reported as a cause of neurologic disease, occurring in 0.5%–6.2% of dengue patients (6–9). Other common viral causes of encephalitis include enterovirus and herpes simplex viruses (HSVs) 1 and 2 (10).
Few data globally are available to guide policy on the prevention, diagnosis, and treatment of CNS infections, and the diversity of definitions for different CNS infection syndromes is confusing (11); some case definitions use clinical criteria only (12,13), and others include additional laboratory variables (10,14). Meningitis (i.e., meningeal infection) and encephalitis (i.e., parenchymal infection) presumably represent a continuum, but the diversity of clinical and laboratory features and etiologies across this wide spectrum is poorly understood. The standard for differentiating encephalitis from meningitis is histopathology, but biopsies and autopsies are rarely performed in Asia.
In Laos, the only comprehensive routine cerebrospinal fluid (CSF) diagnostic service available is in the capital city, Vientiane, at Mahosot Hospital (15–17). After a publication reporting rickettsial and leptospiral pathogens as important causes of CNS infections in Laos (4), we present the results of the full investigation conducted on the causes of CNS infection in this hospital to guide public health policy and treatment guidelines.
Study Site and Patient Recruitment
This study was prospectively conducted (January 2003–August 2011) with inpatients on all wards of Mahosot Hospital in Vientiane (17.959431°N, 102.613144°E, 188 m above mean sea level), an ≈400-bed hospital providing primary, secondary, and tertiary care and admitting ≈2,000 patients/month. We recruited inpatients of all ages for whom diagnostic lumbar puncture was indicated for suspicion of CNS infection because of altered consciousness or neurologic findings and for whom lumbar puncture was not contraindicated. For patient inclusion, we used no formal definition for CNS infection; patient recruitment was at the discretion of the responsible physician, reflecting local clinical practice. We recorded patient history and examination findings on standardized forms.
Ethics Statement
We obtained verbal (2003–2006) or written (2006–2011) informed consent from all recruited patients or close relatives. Ethics clearance was granted by the Ethical Review Committee of the Faculty of Medical Sciences, National University of Laos (Vientiane, Laos), and the Oxford University Tropical Ethics Research Committee (Oxford, UK).
Encephalitis and Meningitis Clinical Case Definitions
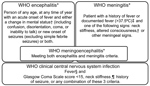
We classified febrile patients meeting the World Health Organization (WHO) criteria for encephalitis or meningitis (Figure 1) (18) as patients with WHO clinical CNS (hereafter WHO CNS) infection. Because of the overlapping WHO case definitions for encephalitis and meningitis, which both include a Glasgow Coma Scale (GCS) score <15 as criteria, we created additional classifications for febrile patients: those with stiff neck; reduced GCS score (<15), seizures, or both; stiff neck and reduced GCS score, seizures, or both; no stiff neck but reduced GCS score, seizures, or both; and stiff neck, a GCS score of 15, and no seizures (Table 1).
Laboratory Tests
Cerebrospinal fluid (CSF) was collected from patients (≈8 mL for adults [defined as patients >15 years of age], ≈3.5 mL for children 1–14 years of age, and ≈2.5 mL for children <1 year of age), and opening pressure was recorded. Venous blood (≈18.5 mL for adults, 10 mL for children 1–14 years of age, and 5.5 mL for children <1 year of age) was drawn on the same day. We aimed to collect ≈2 mL follow-up serum 7–10 days after lumbar puncture. Specimens were transported to the laboratory within ≈30 minutes, and we aliquoted and immediately tested or stored them at −80°C. We submitted all patient samples for a panel of laboratory tests: complete blood count; biochemistry panel; culture; and serologic and molecular assays for a range of bacteria, viruses, parasites, and fungi (Table 2; Appendix). HIV-1 and HIV-2 rapid diagnostic tests were performed when indicated by the physician. Computed tomography brain scan was available starting in 2002 but rarely used, especially for intensive care patients, because of difficulties transferring patients. Magnetic resonance imaging and electroencephalographic facilities were not available.
Interpretation
The confirmed etiology was determined by the results of a panel of diagnostic tests (Table 2), which included tests for the direct detection of pathogens in CSF or blood, specific IgM in CSF, seroconversion, or a 4-fold rise in antibody titer between admission and follow-up serum samples. Pathogen detection was confirmed after critical analysis of test results to rule out possible contamination. When evidence of >1 pathogen was obtained for a patient, we defined the confirmed etiology as detection by direct tests over indirect tests (antibody-based tests) and prioritized CSF detection over blood detection (39). We defined a confirmed co-infection as the direct (or indirect, if only indirect tests were positive) detection of >1 pathogen in the same matrix (CSF or blood).
Statistical Analyses
We compared patients with confirmed bacterial infection (including co-infections involving only bacteria) and patients with confirmed viral infection (including co-infections involving only viruses) with all other patients, excluding those with mixed co-infections (i.e., co-infections with fungi or infections with both bacteria and viruses). We investigated the factors associated with death (died in hospital or discharged moribund), bacterial infection, or viral infection by univariate analysis using the χ2 or Fisher exact test for categorical variables or the Mann-Whitney U test for continuous variables. We analyzed the independent predictors of death, bacterial infection, and viral infection using multivariate logistic regression models. In multivariate analyses, we included all factors having a p value <0.010 in the univariate analysis.
For variables with 6%–20% of the values missing, we executed multiple imputation models using chained equations and used a number of imputations that exceeded the highest proportion of missing values (40). We added age and sex to imputation models as auxiliary variables. We specified the imputation methods as linear for continuous normally distributed variables, logistic for binary variables, ordered logistic for ordinal variables, and predictive mean matching for continuous skewed variables. We performed logistic regression with the dependent variable (death, bacterial or viral infection) and all relevant covariates on each imputed data set and combined results using Rubin rules to take into account the variability in estimates among imputed data sets (41). Only variables that were significant (p<0.050) were retained in the final models. For comparison of analysis methods, we provided the results using the corresponding complete case analysis. We conducted analyses using Stata/SE version 14.0 (StataCorp, https://www.stata.com).
Patients
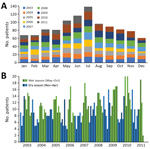
Figure 3. Recruited patients with suspected central nervous system infection, by month, Laos, January 2003–August 2011. A) Total patients recruited by month cumulating all studied years. B) Patients recruited each month of each...
In total, 1,065 inpatients with suspected CNS infection consented to study participation (Appendix Figure 1); 80% were recruited from the pediatric and adult intensive care wards and adult infectious disease ward. On each ward, ≈8 physicians were in charge of patient recruitment. All were general hospital or infectious disease physicians; none were neurologists. We collected information on patient demographics, clinical presentation, and blood and CSF parameters (Table 3–4; Figure 2). More patients were recruited during the rainy season (Figure 3). The median time between admission and follow-up blood collection was 9 (interquartile range [IQR] 6–16) days. One third (33.6%, 358/1,065) were children <15 years of age (Table 3; Appendix Table 2).
Of 476 adults tested for HIV, 118 (24.8%) were seropositive; of 227 children tested, 1 (0.4%) was seropositive. More than half (61.9%, 590/953) of patients had a history or hospital chart evidence of antibiotic use before lumbar puncture. Most (90.8%, 962/1,059) patients had a history of fever or documented admission fever. The median length of fever at admission was 4 (IQR 2–8) days. The most frequent symptoms and signs were headache (88.1%, 787/893); neck stiffness (64.2%, 683/1,064); confusion (57.4%, 608/1,060); GCS score <15 (52.6%, 551/1,047); and vomiting, diarrhea, or both (54%, 575/1,064). All symptoms and signs were more frequent in children than adults (p<0.05), except headache, which was more frequent in adults (p = 0.023). Most (93.6%, 832/889) patients had CSF findings outside reference ranges (elevated CSF white cell count, elevated CSF lactate, elevated CSF protein, low CSF glucose, or any combination of these parameters) (Table 4; Appendix Table 3, Figure 2).

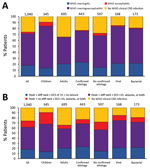
Figure 4. Distribution of clinical presentations in patients with suspected central nervous system infection, by confirmed etiology, Laos, January 2003–August 2011. Analysis per pathogen includes only patients with monoinfections. Other bacteria include 7...
Etiology was confirmed in 450 (42.3%) patients; 413 (38.8%) had monoinfections and 37 (3.5%) co-infections (Appendix Tables 4–8). The identified monoinfections were JEV (8.8%, 94/1,065), Cryptococcus spp. (6.6%, 70/1,065; 9 C. gattii), Orientia tsutsugamushi (2.9%, 31/1,065), dengue virus (2.5%, 27/1,065), Leptospira spp. (2.3%, 25/1,065), Rickettsia spp. (2.3%, 24/1,065), S. pneumoniae (2.1%, 22/1,065), M. tuberculosis (1.9%, 20/1,065), HSV-1 or HSV-2 (1.4%, 15/1,065), human cytomegalovirus (1.1%, 12/1,065), enterovirus (0.9%, 10/1,065), varicella zoster virus (0.6%, 6/1,065), mumps virus (0.5%, 5/1,065), and Plasmodium falciparum (0.4%, 4/1,065). Other bacteria were detected in 48 (4.5%) patients (Figure 4; Appendix Table 5). All samples were negative for West Nile virus, influenza A and B, Henipavirus, and measles virus by PCR. Infection by M. tuberculosis, Cryptococcus spp., or varicella zoster virus was not detected in children (Appendix Table 8). The median age of children with enterovirus infection was 4.5 (IQR 1–11) years and JEV infection 13 (IQR 8–20) years. The proportion of patients with JEV infection was higher for children (14%, 50/358) than adults (6%, 44/707, p<0.001). Significantly more enterovirus patients (80%) than nonenterovirus patients (33%; p = 0.002) were children.
Factors Associated with Bacterial and Viral Infections
We compared patients with single (n = 170) or multiple (n = 5) bacterial infections (excluding co-infections with viruses or fungi) with all other patients (n = 875). Factors significantly associated with bacterial infections on univariate analysis (p<0.01; AppendixTable 9) were included in multivariate analysis. Diabetes (adjusted odds ratio [aOR] 3.1, 95% CI 1.2−7.7), history of fever or fever at admission (aOR 3.9, 95% CI 1.4−11.1), higher serum C-reactive protein (aOR 1.08, 95% CI 1.05−1.11), and higher CSF lactate (aOR 3.5, 95% CI 2.3−5.4) were independent predictors of bacterial infection (AppendixTable 10).
We compared patients with single (n = 169) or multiple (n = 3) viral infections (excluding co-infections with bacteria or fungi) with all other patients (n = 867). Factors significantly associated with viral infections on univariate analysis (p<0.01; Appendix Table 11) were included in multivariate analysis. Neck stiffness (aOR 1.9, 95% CI 1.3−2.8) and higher hematocrit (aOR 1.4, 95% CI 1.1−1.9) were associated with viral infection, whereas higher CSF lactate (aOR 0.3, 95% CI 0.1–0.5), older age (aOR 0.8, 95% CI 0.7–0.9), and longer interval between admission and lumbar puncture (aOR 0.9, 95% CI 0.8–1.0) were negatively associated with viral infection (Appendix Table 12).
Relationships between Clinical Presentation and Etiology
In total, 771 (74.1%) of 1,040 patients had WHO CNS infection; 44.2% of these patients had confirmed etiologies compared with 37.9% of patients not fulfilling WHO CNS infection criteria (p = 0.063; Appendix Table 13). Because of the considerable overlap between the WHO encephalitis and meningitis definitions, 551 (71.5%) patients were classified as having meningoencephalitis. Therefore, we analyzed the frequency of neck stiffness, reduced GCS score, and seizures among febrile patients with clinical CNS infection (Table 1).
When comparing viral and bacterial infections, we observed no significant differences (p>0.05) in the proportions of encephalitis and meningitis syndromes, although differences were observed for some specific etiologies (Figure 4). In total, 90 (53.6%) febrile patients with viral infection and 86 (49.7%) with bacterial infection had neck stiffness and reduced GCS score, seizures, or both; 17 (10.1%) patients with viral infection and 16 (9.3%) with bacterial infection had reduced GCS score, seizures, or both without neck stiffness; and 36 (21.4%) patients with viral infection and 38 (22.0%) with bacterial infection had neck stiffness, a GCS score of 15, and no seizures (Figure 4). We obtained similar results using the WHO definitions. In total, 25 (14.9%) patients with viral infection and 33 (19.1%) with bacterial infection did not fulfill the WHO CNS infection definition.
In comparison with the distribution of syndromes observed for all patients, the distribution in patients with some etiologies were significantly different (p<0.05). Of the 89 JEV patients with WHO CNS infection, 75.3% had fever; neck stiffness; and reduced GCS score, seizures, or both. Of the 26 O. tsutsugamushi patients with WHO CNS infection, 50% had fever, neck stiffness, a GCS score of 15, and no seizures. Of note, almost half (47.8%) of the patients with cryptococcal infection did not fulfill the definition for WHO CNS infection, and of the 36 who did, 55.6% had fever, neck stiffness, a GCS score of 15, and no seizures.
Risk Factors for Death
Of 893 patients, 235 (26.3%) died, including those discharged moribund; we compared them to the 658 (73.7%) patients discharged alive and not moribund. For factors significantly associated with death (p<0.01; Appendix Table 14) in the univariate analysis, we conducted multivariate analysis. The variables strongly associated with death were higher CSF lactate (aOR 1.1, 95% CI 1.0–1.1) and reduced GCS score (aOR 0.8, 95% CI 0.8–0.9). Patients with viral infection were less likely to die than those with other diagnoses (aOR 0.4, 95% CI 0.3–0.7) (Appendix Table 15). Diabetes and hyperglycemia (glucose >7.7 mmol/L) at admission were not associated with death.
Indications for Antibiotic Treatment
In total, 56 patients (12.4% of the 450 patients with confirmed etiologies) were infected with bacteria treatable by ceftriaxone and 64 patients (14.2% of the patients with confirmed etiologies) with bacteria treatable by doxycycline but not ceftriaxone (Table 5). Twenty-eight patients were infected with a Leptospira spp. treatable by ceftriaxone or doxycycline, but 2 were co-infected with O. tsutsugamushi not treatable by ceftriaxone. Of 142 patients infected by bacteria treatable by ceftriaxone or doxycycline, 89 (62.7%) received appropriate treatment, 17% (13/77) of whom died. In comparison, 25% (12/48) of the patients who did not receive appropriate treatment died (p = 0.270).
Including the 450 patients with confirmed diagnoses, we analyzed the criteria for bacterial meningitis commonly considered when making decisions on antibiotic treatment: elevated CSF white cell count, elevated CSF lactate, elevated CSF protein, decreased CSF glucose, reduced GCS score, turbid CSF, and neck stiffness. A low percentage (<23%) of patients with any 1 of these criteria (except turbid CSF, 38.8%) or a combination of these criteria had bacterial infections treatable by ceftriaxone or doxycycline (Appendix Table 16). Furthermore, only 1 combination of criteria (elevated C-reactive protein, elevated CSF protein, or elevated CSF lactate or any combination of these criteria) could identify all patients infected with bacteria treatable by ceftriaxone (Table 5). However, because only 5% of our patient series did not display this combination of criteria, none of the analyzed clinical and biological results can be reliably used to guide decisions on antibiotic use.
Etiology was confirmed in 42.3% of patients with suspected CNS infection, consistent with regional published data (Appendix Table 1); 16.2% had viral infections, and 16.4% had bacterial infections. We observed no significant differences in the distribution of clinical encephalitis and meningitis syndromes by bacterial or viral etiology; the most common infections in this patient population were JEV (8.8%) and Cryptococcus spp. (6.6%).
The results of this study provided evidence for the implementation of pneumococcal immunization in 2011 and JEV immunization in 2013 in Laos (16,17). Although the main etiology reported among patients with suspected CNS infection was JEV, this finding might be an overestimate; we have noted that the detection of JEV IgM in CSF has low predictive value (44). On the other hand, bacterial causes were probably underestimated; 61.9% of patients were known or thought to have received an antibiotic before lumbar puncture, potentially rendering bacteria uncultivable or reducing the bacterial load below the threshold needed for molecular detection.
The mortality rate we report in our study (26.3%) was higher than those reported in similar studies in neighboring countries (≈10%; Appendix Table 1). Ineffective patient management or inappropriate treatment through lack of previous local data might have caused this higher mortality rate. The epidemiology of CNS infection varies by geography; therefore, regional evidence should be used to build regional policies on prevention, diagnosis, and treatment of these infections.
In this study, 17% (119/703) of the patients tested were HIV seropositive. The highest proportion of HIV-seropositive patients was among those with cryptococcal infection (79%; AppendixTable 7). However, only 66% (703/1,065) of patients were tested. More patients need to receive HIV testing in Laos, and more investigations on the relationship between HIV and other infections are needed.
Our study had a number of limitations, including the partial use of stored samples; missing values; a low frequency of computed tomography brain scans and HIV testing; and a lack of magnetic resonance imaging, brain or postmortem examination, and diagnostics for autoimmune and eosinophilic CNS disease (45,46) and other pathogens (e.g., Toxoplasma gondii, Mycoplasma spp., and Zika virus). The absence of strict criteria for the inclusion of patients could have resulted in recruitment bias; however, the data reflect real-life medical practice. The proportion of patients who declined lumbar puncture is unknown. Almost all patients (93.6%) had CSF findings outside reference ranges. Although published data on this combined index are few, the proportion of patients with abnormal CSF findings is generally lower in routine practice (e.g., <40% at La Timone Hospital, Marseille; L. Ninove and J. Fromonot, La Timone Hospital, pers. comm., March 2017). This finding suggests a relatively low frequency of lumbar puncture at Mahosot Hospital, reflecting current practice but representing an unknown proportion of all patients admitted with CNS disease. The sample size was too small for a comparison of mortality rates between treated and nontreated patients.
Although CNS infection is a global public health burden, global consensus on the case definition is lacking (Appendix Table 17). In clinical studies, encephalitis and meningitis have been studied separately or together (11), and CSF findings might or might not be taken into account (e.g., CSF findings are not part of the WHO criteria). There is confusion regarding the clinical and laboratory definitions of encephalitis and meningitis, so we suggest pairing clinical, laboratory, or clinicolaboratory with these terms to reduce confusion. Further, altered consciousness and altered mental status, shared by encephalitis and meningitis in many definitions, are undefined in the WHO definitions. We used GCS score <15 to define both objectively, but this practice resulted in considerable overlap in clinical definitions: 71.4% of patients had WHO-defined meningitis and 53% WHO-defined meningoencephalitis. When we restricted the definition of meningitis to the presence of fever and neck stiffness, 61.9% of patients fulfilled those criteria; 43.6% had neck stiffness combined with low GCS score, seizures, or both.
Studies on the clinical and etiologic characteristics of patients requiring lumbar puncture in Asia have usually focused on particular pathogens or just meningitis or encephalitis (Appendix Table 1) (47). Although needed for treatment trials and pathophysiologic research, our data call into question the validity of defining criteria for patient management differently between encephalitis and meningitis (Figure 4; AppendixTable 13). In Laos, evidence suggests that brain (encephalitis) and meningeal (meningitis) infections have no clear distinguishable clinical manifestations relating to the responsible pathogen and that these classifications should be used with caution in the Asia tropics for guiding patient management.
We found that history of diabetes was independently associated with bacterial CNS infection. Indeed, some evidence suggests that diabetes is a risk factor for bacterial CNS disease (48) and poor outcome in tuberculous meningitis (49). In univariate analysis, higher blood glucose level was also associated with bacterial infection (p<0.001). Of 237 patients with hyperglycemia at admission (>7.7 mmol/L), 16 (6.8%) had a history of diabetes and 164 (69.2%) did not. Without convalescent glucose and hemoglobin A1c assays, however, we were unable to distinguish hyperglycemia resulting from severe disease or undiagnosed diabetes that might have predisposed to CNS infection. Intensive euglycemia management is difficult; it can lead to hypoglycemia, especially in unconscious patients, and requires skilled dedicated nursing that is not available in hospitals in rural Asia. Whether such intensive therapy would save lives remains uncertain, but the development of an inexpensive computerized algorithm technology for resource-poor settings to facilitate safe euglycemia management (50) should be a priority for investigation of efficacy. The burgeoning global prevalence of diabetes calls for research regarding the relationship between hyperglycemia and CNS infections and optimizing their combined management (48).
Our data suggest that patient survival could be improved through 2 patient management interventions, the implementation of antibiotic use guidelines and strengthening of high-dependency units. The finding that poor outcomes were associated with a decreased GCS score at admission suggests that high-dependency units (a likely cost-effective intervention) could be used to enhance supportive care for unconscious patients with CNS infection. Creating these units and incorporating them into care could improve outcomes and reduce the burden of intensive care unit treatment for these patients. More investigation is needed on the efficacy and cost-effectiveness of high-dependency units in different contexts.
Ceftriaxone is conventionally used in Laos as a first-line treatment for CNS bacterial infection but lacks efficacy for emerging rickettsial pathogens, for which doxycycline is recommended (4). Because delays in antibiotic therapy could result in severe consequences for patients, the decision for administering these drugs is made on the basis of clinical signs and laboratory results at admission. However, in Laos, we found that no variable, even in combination, could permit objective selection of appropriate antibiotics. Therefore, the administration of early first-line empiric treatment with ceftriaxone and doxycycline for all patients with suspected CNS infection might save patient lives in Laos and elsewhere in rural Asia (4).
Dr. Dubot-Pérès is a virologist serving as the Head of Partnerships and Programmes of South Countries for the Unité des Virus Émergents (Aix-Marseille University, IRD 190, INSERM 1207, IHU Méditerranée Infection) in Marseille, France. She is an Honorary Visiting Research Fellow at the Nuffield Department of Medicine, University of Oxford, Oxford, UK, and the Head of Virology at the Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit in Vientiane, Laos. Her main research topics of interest are the study of undifferentiated fever, dengue epidemiology, infections of the CNS, and respiratory infections.
Acknowledgments
We thank the patients, Bounthaphany Bounxouei (Associate Professor and Director), and staff of Mahosot Hospital, especially the microbiology laboratory staff and the ward staff for their technical help and support. We also thank Bounnack Saysanasongkham (Associate Professor and Director of Department of Health Care, Ministry of Health) and Bounkong Syhavong (Associate Professor and Minister of Health), Laos, for their kind help and support. We thank the staff of the Meningococcal Reference Unit of the Health Protection Agency (Manchester, UK) for N. meningitidis typing and the staff of the Respiratory and Vaccine Preventable Bacteria Reference Unit of Public Health England (Colindale, UK) for Haemophilus influenzae typing. We also thank Mavuto Mukaka for his help with statistical analysis and Nicholas Day for comments on the paper.
This work was supported by the Wellcome Trust of Great Britain, the Institute of Research for Development, Aix-Marseille University, and the European Union’s Horizon 2020 research and innovation program European Virus Archive global (grant agreement no. 653316).
References
- World Health Organization. Japanese encephalitis. 2015 Dec 31 [cited 2018 Jun 6]. https://www.who.int/en/news-room/fact-sheets/detail/japanese-encephalitis
- Moore CE, Sengduangphachanh A, Thaojaikong T, Sirisouk J, Foster D, Phetsouvanh R, et al. Enhanced determination of Streptococcus pneumoniae serotypes associated with invasive disease in Laos by using a real-time polymerase chain reaction serotyping assay with cerebrospinal fluid. Am J Trop Med Hyg. 2010;83:451–7. DOIPubMed
- Moore CE, Blacksell SD, Taojaikong T, Jarman RG, Gibbons RV, Lee SJ, et al. A prospective assessment of the accuracy of commercial IgM ELISAs in diagnosis of Japanese encephalitis virus infections in patients with suspected central nervous system infections in Laos. Am J Trop Med Hyg. 2012;87:171–8. DOIPubMed
- World Health Organization. Recommended standards for surveillance of selected vaccine-preventable diseases. 2003 [cited 2018 Jun 6]. http://www.measlesrubellainitiative.org/wp-content/uploads/2013/06/WHO-surveillance-standard.pdf
- Cole JR Jr, Sulzer CR, Pursell AR. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol. 1973;25:976–80.PubMed
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, et al. Rapid detection of west nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–71.PubMed
- Kessler HH, Mühlbauer G, Rinner B, Stelzl E, Berger A, Dörr HW, et al. Detection of Herpes simplex virus DNA by real-time PCR. J Clin Microbiol. 2000;38:2638–42.PubMed
- Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley and Sons; 1987.
- Bennett JE, Dolin R, Blaser MJ. Principles and practice of infectious diseases. 8th ed. Philadelphia: Elsevier Saunders; 2014.
- Eamsobhana P. Angiostrongyliasis in Thailand: epidemiology and laboratory investigations. Hawaii J Med Public Health. 2013;72(Suppl 2):28–32.PubMed
Figures
Tables
Cite This ArticleOriginal Publication Date: 4/9/2019
1Deceased.
No comments:
Post a Comment