A new DRUG TRIALS SNAPSHOT is now available.

GIVLAARI is a drug for the treatment of adults with acute hepatic porphyria (AHP).
Acute hepatic porphyria is a rare metabolic disease. It results from a buildup of porphyrin molecules which are formed during the production of heme (which helps bind oxygen in the blood). AHP is characterized by acute attacks which can lead to severe pain and paralysis, respiratory failure, seizures and mental status changes.
GIVLAARI is an injection. It is given by a healthcare professional under the skin (subcutaneously) once a month.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
Drug Trials Snapshots: GIVLAARI
GIVLAARI (givosiran)
giv lah' ree
Alnylam Pharmaceuticals
Approval date: November 20, 2019
giv lah' ree
Alnylam Pharmaceuticals
Approval date: November 20, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
GIVLAARI is a drug for the treatment of adults with acute hepatic porphyria (AHP).
Acute hepatic porphyria is a rare metabolic disease. It results from a buildup of porphyrin molecules which are formed during the production of heme (which helps bind oxygen in the blood). AHP is characterized by acute attacks which can lead to severe pain and paralysis, respiratory failure, seizures and mental status changes.
How is this drug used?
GIVLAARI is an injection. It is given by a healthcare professional under the skin (subcutaneously) once a month.
What are the benefits of this drug?
Patients who received GIVLAARI experienced fewer porphyria attacks (about 1.9 attacks) in a 6-month period, compared to patients who received placebo (about 6.5 attacks).
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
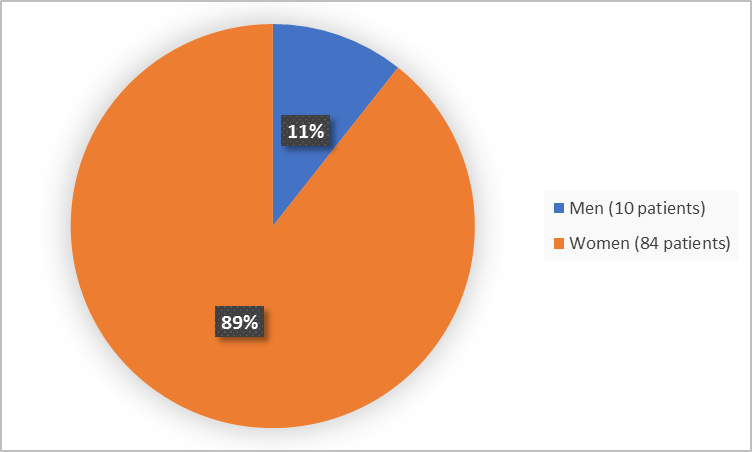
- Sex: The majority of patients were women. Differences in how GIVLAARI worked between men and women could not be determined.
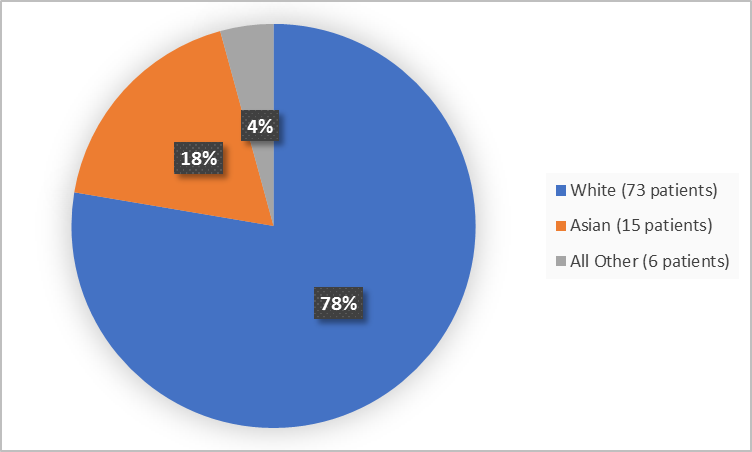
- Race: The majority of patients were White. The number of patients in other races were limited; therefore, differences in how GIVLAARI worked among races could not be determined.
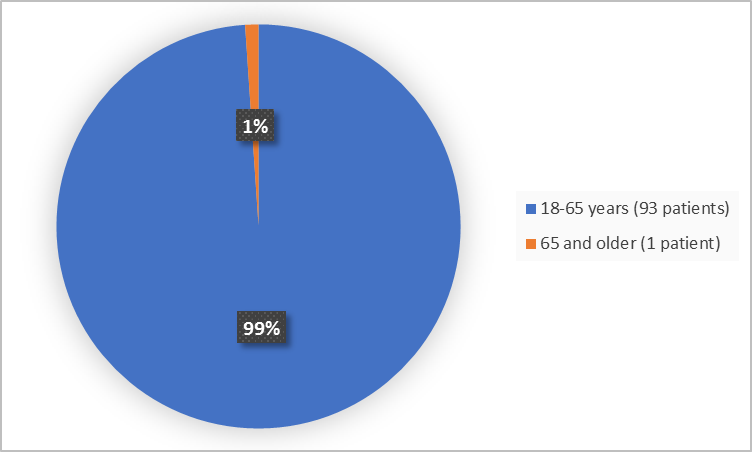
- Age: The majority of patients were adults between 18-65 years of age. There were not enough patients older than 65 years to determine whether there was any difference in how GIVLAARI worked between older and younger patients.
What are the possible side effects?
GIVLAARI may cause life-threatening allergic reactions, serious liver and kidney injury and injection site reactions.
The most common side effects of GIVLAARI include nausea and injection site reactions.
Were there any differences in side effects among sex, race and age?
- Sex: The majority of patients were women. Differences in the occurrence of side effects between men and women could not be determined.
- Race: The majority of patients were White. The number of patients in other races were limited; therefore, differences in occurrence in side effects among races could not be determined.
- Age: The majority of patients were adults between 18-65 years of age. There were not enough patients older than 65 years to determine whether there was any difference in side effects between older and younger patients.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved GIVLAARI based on evidence from one clinical trial (Trial 1/ NCT03338816) of 94 patients with AHP. The trial was conducted at 36 sites in the United States, Canada, Europe, Asia, Australia, and Mexico.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
All Other includes Black or African American, Native Hawaiian or Other Pacific Islander and Mixed race
FDA Review
Figure 3 summarizes the percentage of patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA Review
How were the trials designed?
There was one trial that evaluated the benefit and side effects of GIVLAARI in patients with AHP and a minimum of 2 porphyria attacks requiring hospitalization, urgent healthcare visit, or intravenous hemin (an approved drug for AHP attacks) administration at home in the 6 months prior to trial entry.
Patients received at random either GIVLAARI or placebo injections once monthly for 6 months. Neither the patients nor the healthcare providers knew which treatment was given until the end of the trial.
The benefit of GIVLAARI compared to placebo was assessed by measuring by the rate of porphyria attacks that required hospitalizations, urgent healthcare visit, or intravenous hemin administration at home.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
No comments:
Post a Comment