Drug Trial Snapshot: RECARBRIO

RECARBRIO is a drug for adults who do not have additional options to treat:
- Complicated urinary tract infection (cUTI) including infection of the kidneys (pyelonephritis) caused by specific bacteria or,
- Complicated intra-abdominal infections (cIAI) (serious infections that extend into the intra-abdominal cavity and require treatment in the hospital) caused by specific bacteria.
RECARBRIO is a drug administered by a health care professional directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV, infusion. It takes about 30 minutes to receive a RECARBRIO infusion.
See more Drug Trials Snapshots or contact us with questions at Snapshots@fda.hhs.gov.
RECARBRIO (imipenem, cilastatin, and relebactam)
reh-CAR-bree-oh
Merck Sharp & Dohme Corp.
Approval date: July 16, 2019
reh-CAR-bree-oh
Merck Sharp & Dohme Corp.
Approval date: July 16, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
RECARBRIO is a drug for adults who do not have additional options to treat:
- Complicated urinary tract infection (cUTI) including infection of the kidneys (pyelonephritis) caused by specific bacteria or,
- Complicated intra-abdominal infections (cIAI) (serious infections that extend into the intra-abdominal cavity and require treatment in the hospital) caused by specific bacteria.
RECARBRIO is a combination of the previously approved drugs (imipenem/cilastatin and a new drug (relebactam). RECARBRIO should only be used when the infection is caused by bacteria or strongly suspected to be caused by bacteria.
How is this drug used?
RECARBRIO is a drug administered by a health care professional directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV, infusion. It takes about 30 minutes to receive a RECARBRIO infusion.
RECARBRIO is given every 6 hours for 4 to 14 days.
What are the benefits of this drug?
In patients who have few or no other treatment options, RECARBRIO treats cIAI and cUTI.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The trials that evaluated RECARBRIO provided limited benefit information. Therefore, differences in how the drug worked among sex, race, and age subgroups could not be determined.
What are the possible side effects?
RECARBRIO may cause serious side effects such as severe allergic reactions, seizures, and antibiotic-associated diarrhea called clostridium difficile.
The most common side effects of RECARBRIO are diarrhea, nausea, headache, vomiting and abnormal liver tests.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar among men and women.
- Race: The majority of patients were White. The number of patients in other races was limited. Therefore, differences in the occurrence of side effects could not be determined.
- Age: The occurrence of side effects was similar among patients younger and older than 65 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
RECARBRIO was studied in two clinical trials (Trial 1/NCT01505634, Trial 2/NCT01506271) of 430 patients with cUTI or cIAI. The trials were conducted in Europe, South America, United Sates, Asia Pacific, Africa, and Mexico.
The FDA primarily considered previous findings of the efficacy and safety of imipenem/cilastatin in the treatment of cIAI and cUTI as well as the evidence from the laboratory and animal studies showing the contribution of relebactam.
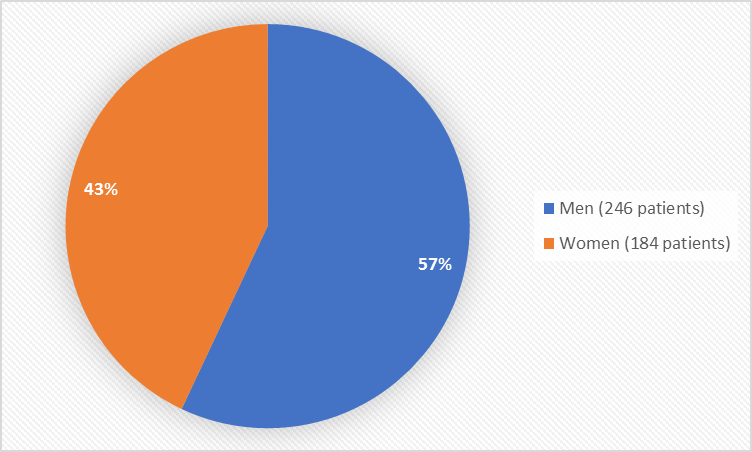
Figure 1 summarizes how many men and women were in the clinical trials used to evaluate safety.
Figure 1. Baseline Demographics by Sex (safety population)
FDA Review
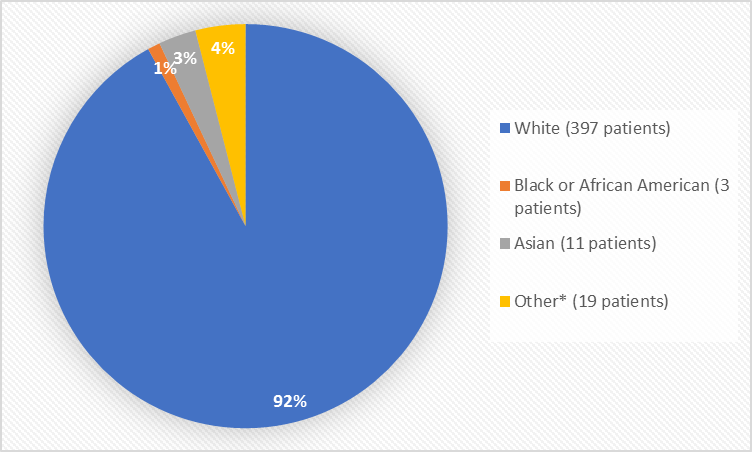
Figure 2. Baseline Demographics by Race (safety population)
*Other includes American Indian, or Alaska Native
FDA Review
Table 1. Demographics of Trials by Race (safety population)
Race | Number of Patients | Percentage of Patients |
White | 397 | 92% |
Black or African American | 3 | 1% |
Asian | 11 | 3% |
American Indian or Alaska Native | 2 | Less than 1 |
Other | 17 | 4% |
FDA Review
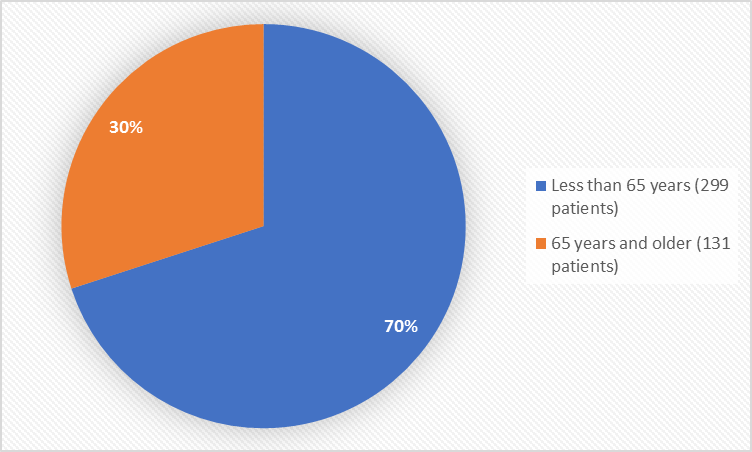
Figure 3 summarizes the percentage of patients by age group in the clinical trials used to evaluate safety.
Figure 3. Baseline Demographics by Age (safety population)
FDA Review
How were the trials designed?
Trial 1 enrolled adult patients hospitalized with cUTI. Trial 2 enrolled adult patients hospitalized with cIAI that required surgery or drainage. In both trials, patients were assigned to either imipenem/cilastatin with varying doses of relebactam or imipenem/cilastatin with placebo intravenously, every 6 hours for 4 to 14 days. Neither the patients nor the investigators knew which treatment was being given until after the trial was completed.
The trials provided data primarily for evaluation of side effects.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
No comments:
Post a Comment